Abstract
Introduction: Gemtuzumab ozogamicin (GO; MylotargTM) was approved by the US Food and Drug Administration in 2017 and the European Medicines Agency in 2018 in combination with standard of care (SOC) chemotherapy (daunorubicin/cytarabine) for the treatment of de novo acute myeloid leukemia (AML). Although initial remission rates for de novo AML are high, overall survival (OS) is low; however, a proportion of patients experience long-term remission (>5 years) and can be considered cured. Statistically cured populations can be estimated by fitting mixture cure models (MCMs) to survival data, a method that takes into account background survival rates. The objective of this research was to assess and compare the statistical cure rates with GO plus SOC vs SOC alone among adult patients with de novo AML.
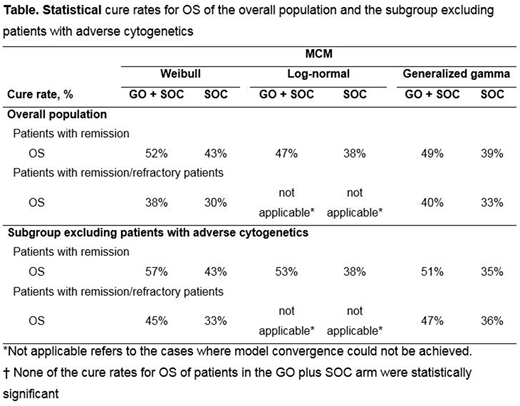
Methods: MCMs (Weibull, lognormal, and gamma) were fitted to patient-level data from the phase 3 ALFA-0701 trial on event-free survival (EFS) and OS of patients with remission (GO plus SOC: n=100; SOC alone: n=96), and OS of all patients (with remission and refractory; GO plus SOC: n=135; SOC alone: n=136). As refractory patients do not experience remission, this methodology would not provide clinically meaningful results since they are unlikely to experience a cure. Analyses for the subgroup excluding patients with adverse cytogenetics were also explored. For the GO plus SOC arm, P values <0.05 deemed a statistically significant difference in cure rates between treatment arms. The analyses were implemented in STATA (version 13) statistical package, which accounts for general population mortality in the MCM parametrization.
Results: The statistical cure rates of MCMs on EFS for patients with remission for the GO plus SOC and SOC alone arms varied (depending on the distribution) from 34% (P=0.018) to 38% (P=0.018) and from 17% (P=0.008) to 21% (P<0.001), respectively. For the subgroup excluding patients with adverse cytogenetics, EFS cure rates varied from 36% (P=0.007) to 41% (P=0.007) for patients treated with GO plus SOC and from 14% (P=0.113) to 20% (P<0.001) for patients on SOC alone. For OS of patients in remission and patients in remission/refractory states, the GO plus SOC arm demonstrated a trend towards higher statistical cure rates compared to SOC alone. (Table)
Conclusions: In both subgroups explored, patients in remission treated with GO plus SOC chemotherapy demonstrated improved cure rates in EFS of newly diagnosed patients with de novo AML compared to patients treated with SOC alone. For OS of patients with remission, GO plus SOC displayed numerically higher (but not statistically significant) cure rates compared with SOC patients. Findings were similar in the overall population that included remission and refractory patients and for the subgroup excluding patients with adverse cytogenetics
These analyses are limited by the relatively small number of patients in the context of a randomized trial, making it difficult to ascertain if there are sufficient data to accurately estimate cure fractions. Nevertheless, expert validation of cure rates, long-term survival projections, and relative differences between treatment arms suggest the results are reliable. Although a novel survival modeling method, MCMs provided a good visual fit to the EFS and OS in remission data from the ALFA-0701 trial, in part because MCMs account separately for the proportion of cured and uncured patients who experience long-term survival.
Muresan:Ingress-health Nederland BV: Employment. Mamolo:Pfizer: Employment, Equity Ownership. Cappelleri:Pfizer: Employment, Equity Ownership. Mokgokong:Pfizer Inc: Equity Ownership. Palaka:Pfizer Inc: Employment, Equity Ownership. Hills:Daiichi Sankyo: Consultancy, Honoraria. Latimer:Astra Zeneca: Consultancy; Merck: Consultancy; Pfizer: Consultancy; BMS: Consultancy; Janssen: Consultancy; Bluebirdbio: Consultancy. Heeg:Ingress-health Nederland BV: Employment, Equity Ownership, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal